
Expressions of Interest for the Spinal Injury Project (SIP) stage two Intensive Rehabilitation Trial are now open!
This stage two trial is for people living with chronic spinal cord injury in Australia who have had minimal rehabilitation in the last two years. This trial is important in defining the protocol that will be paired with the cell transplantation therapy that is being developed by the Spinal Injury Project team at Griffith University. The trial’s purpose is to examine whether intensive rehabilitation therapy can be safely and effectively delivered to people who are not experienced with long-term or intensive rehabilitation activities. It will also assess if the program improves functional recovery for this cohort of people, as well as improves overall health and social outcomes.

Who is eligible?
This study seeks 5 participants based in South-East Queensland/Northern New South Wales, who are at least 18 years of age and who have sustained a traumatic spinal cord injury at least 12 months ago with ASIA impairment scale grade A or B (documented by an ISNCSCI/ASIA exam performed by a qualified practitioner within the last 6 months).
Participants will have completed their primary in-patient rehabilitation.
Participants will have not exercised more than 2 times a week, 40 weeks a year (or no more than 80 sessions per year), at a specialised rehabilitation centre in the last two years and will be vaccinated against COVID-19 and have had their COVID-19 booster (if eligible).
Exclusion criteria are those that limit people’s ability to participate or pose health risks, including, but not limited to:
- significant concomitant central nervous system or other disorders that limit ability to exercise
- recent major trauma or surgery within the last 6 months
- existing stage 3 or 4 pressure ulcer
- other serious medical conditions
- uncontrolled neuropathic pain
- autonomic dysreflexia without a management plan
- symptomatic or radiologically demonstrated ischaemic heart disease
- chronic obstructive pulmonary disease
- contraindications for functional electrical stimulation (FES)
- pregnancy
- currently participating in other trials (medical or therapeutic interventions).
How does the Trial work?
Expressions of interest will be collected by an independent third party and a shortlist of 10 – 15 eligible applicants will be provided to the study team for pre-screening. These applicants, if in agreement, will be volunteering for the pre-screening process. This involves signing a pre-screening consent form informing them that they are not guaranteed a place on the study and will be reviewed for eligibility and suitability for the study. Review of medical records and discussions with the applicants will take place at this stage. To ensure the process is as fair as possible, applicants with similar profiles/injuries who are suitable for the study will then be randomly selected to cover the diverse nature of spinal cord injury. Five participants will then be selected by the principal investigator.
The recruitment period will close when we have 5 suitable candidates.
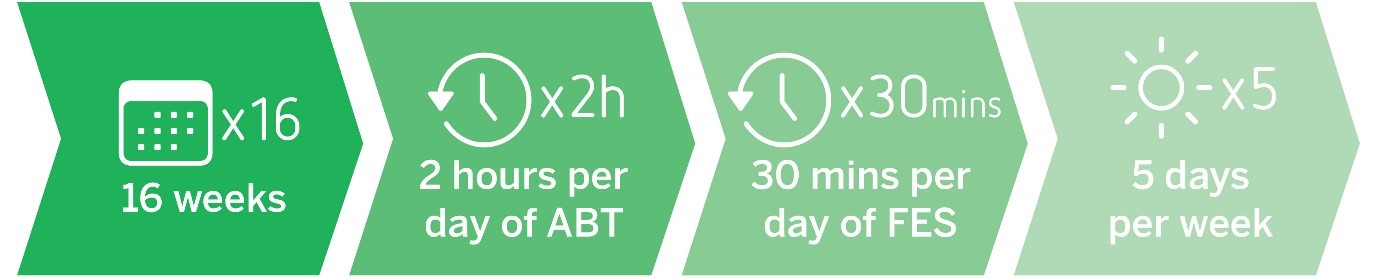
The participants will undertake a 16-week rehabilitation program consisting of 2 hours of activity-based therapy plus 30 min of FES sessions per day, 5 days per week for 16 weeks. These sessions will be run at Making Strides, a specialised rehabilitation centre, located on the Gold Coast, Queensland.
Throughout the trial, functional, medical and psychosocial assessments will be conducted to determine participants’ outcomes.
This Trial, which is funded by The Perry Cross Spinal Research Foundation and sponsored by Griffith University, is part of the broader Spinal Injury Project conducted at the Menzies Health Institute Queensland and the Griffith Institute for Drug Discovery (Griffith University).
If you are interested in participating in this Trial, please complete your expression of interest.
Expressions of Interest for the Spinal Injury Project (SIP) stage two Intensive Rehabilitation Trial
The recruitment period will close when we have 5 suitable candidates.
Thank you for your interest in the SIP Intensive Rehabilitation Trial. A friendly member of the team will contact you shortly.


