Spinal Injury Project

You have the power to change a life today
Spinal Injury Project (SIP)
The Perry Cross Spinal Research Foundation supports and funds The Spinal Injury Project (SIP), which is based at the Menzies Health Institute Queensland (MHIQ) and the Griffith Institute for Drug Discovery (GRIDD) at Griffith University.






The Spinal Injury Project is in pursuit of a cure and is working with a laser focus towards the ultimate goal – a Human Clinical Trial.
This ground-breaking, world first project was pioneered by 2017 Australian of the Year, Emeritus Professor Alan Mackay-Sim and involves the transplantation of the patient’s own olfactory ensheathing cells (OECs) from the nose into the spinal cord. This experimental treatment has shown promise in previous human clinical trials and now needs further refinement.
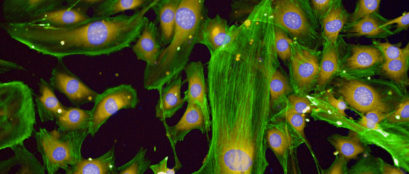
The SIP team is reinventing and rethinking how cells can grow leading to the creation of new cell products. By combining advanced cell purification techniques and engineering, the team is designing three-dimensional nerve bridges that will help regenerate the spinal cord.
These 3D cell constructs use newly invented, award winning technology involving 3D printed templates. This technology generates 3D nerve bridges that can be used to repair traumatic injuries. This methodology ensures patients receive optimal restoration of motor and sensory function and outcomes are as consistent as possible.
This approach has recently been successfully tested in pre-clinical models and has shown promising functional outcomes. This incredible approach has the potential to result in the first widely available treatment for spinal cord injury and it is being developed here in Australia.
Learn more about the Rehabilitation Project
Join us for a Virtual Tour of the Lab
Download the Spinal Injury Project project outline
Find out the latest news on the Spinal Injury Project (SIP)
Find out the latest on the Spinal Injury Project (SIP)
Currently Funded Projects
- Institution:Griffith University
- Lead Researcher:Prof James St John
- PCSRF funding period:2025 - 2028
- Total Funds Committed:$1,700,000

- Institution:Griffith University
- Lead Researcher:Prof James St John
- PCSRF funding period:2024 - Ongoing
- Total Funds Committed:$15,300,000

- Institution:Griffith University
- Lead Researcher:Prof James St John
- PCSRF funding period:2023 - 2026
- Total Funds Committed:$1,520,000

- Institution:Griffith University
- Lead Researcher:Prof James St John
- PCSRF funding period:2023 - Ongoing
- Total Funds Committed:$400,000

- Institution:Griffith University
- Lead Researcher:Prof James St John
- PCSRF funding period:2023 - 2026
- Total Funds Committed:$5,400,000

- Institution:Griffith University
- Lead Researcher:Prof. James St John
- PCSRF funding period:2018 – 2025 (Ongoing)
- Total Funds Committed:$430,252

Clinical Trial Updates
Thankyou for your interest in the Olfactory Ensheathing Cell Transplantation to Repair Spinal Cord Injury Project (SIP)

The Perry Cross Spinal Research Foundation is currently raising funds and awareness to support this project being conducted at Griffith University, under the supervision of Assoc Professor James St John. This exciting research project was pioneered by the 2017 Australian of the Year Professor Alan Mackay-Sim.
At this stage we are collecting details from people that are interested in taking part in the trials, however we are unsure of the timeline for recruitment or any inclusion criteria.
In the meantime, you can help us generate urgent support and awareness for this ground-breaking project by following us on social media and helping to spread the word about the project. Every bit of awareness and support will help us reach our goal – a cure for paralysis for all!
Latest PCSRF news
Griffith University Clinical Trial – Frequently Asked Questions
Important Update – Nerve Bridge Transplantation and Rehabilitation Human Clinical Trial
Human Trial Update from Prof. James St John
International Spinal Research Trust Meeting
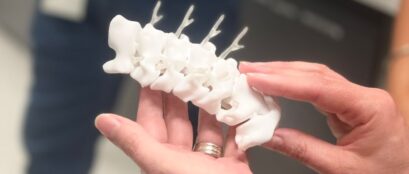
3D Replica Model of Spinal Cord
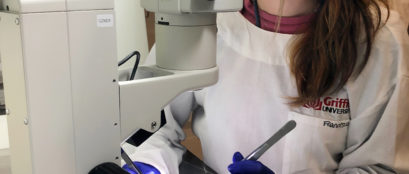
Cutting edge microscope to help find a cure for paralysis
Cell and Nerve Bridge Production Team – Making Progress
Research Open Day at Griffith University
Spinal Injury Project Partnership Prospectus
New Drug Therapy having an impact
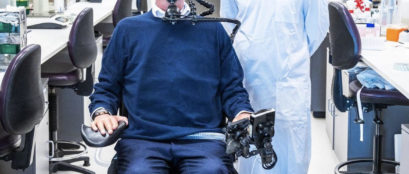
Human Clinical Trial Equipment Purchase
Intensive Prehabilitation Trial is underway
Competitive Grant Secured
Prestigious Research Fellowship awarded to Dr Mo Chen
Funding Boost for Spinal Injury Project Research Team
Exciting news!!
Spinal Cord Community Survey
August Research Update
In pursuit of a cure
Research Open Day at Griffith University
Meet the SIP Team – Dr Heidi Walkden
Meet the SIP Team – Dr Yu-Ting (Tammy) Tseng
Meet the SIP Team – Francesca Oieni
Meet the SIP Team – Chenying Yang
Treatment For Paralysis A Step Closer
Dr Matt & Dr Mike on Spinal Cord Injury
Meet Dr Indra Neil Choudhury
Griffith University biomedical researchers take home top award
SIP Project gets $5.7m boost!
Australasian Neuroscience Society Conference
New Funding for Nerve Bridge Research
SIP researchers to present at SLAS 2020 Conference in San Deigo, California
Clinical Trial Update – Spinal Injury Project
New Funding Announcement!
Meet the SIP Team – Dr Tanja Eindorf
Meet the SIP Team – Dr Ronak Reshamwala
Meet the SIP Team – Dr Mo Chen
UPDATE: Repairing the Injured Spinal Cord with Olfactory Glia Transplantation
Meet the SIP Team – Assoc Prof Jenny Ekberg
People living with SCI and SCI carers wanted to complete survey
Interested in helping to raise funds for this project?
There are many ways you can help